Have you ever been stumped by the relationship between solvents and reaction mechanisms? Well, I'm currently in that situation with acetone and SN1/SN2 reactions. I'm desperate to figure out: Does acetone mean SN1 or SN2? Decoding the impact of acetone on SN1 and SN2 reaction types. I've read that polar protic solvents often favor SN1 reactions, and polar aprotic solvents are more conducive to SN2. But where does acetone fit in? Is it polar enough to have a significant impact on the reaction mechanism? And how does its structure contribute to its effect on these reactions? I'm really looking forward to getting some insights.
Want to Figure Out: Does Acetone Mean SN1 or SN2? Decoding the Impact of Acetone on SN1 and SN2 Reaction Types
Related Encyclopedia
Related Products More >
-
- 96-26-4
- CNY Request For Quotation
-
- 96-26-4
- CNY Request For Quotation
-
- CNY Request For Quotation
-
- CNY Request For Quotation
-
- 67-64-1
- CNY 2000.0000
- 1ton
-
- 51364-51-3
- Request For Quotation
- 100g
-
- 51364-51-3
- Request For Quotation
- 10g
-
- 51364-51-3
- Request For Quotation
- 1kg



 沪ICP备2021018848号-5
沪ICP备2021018848号-5

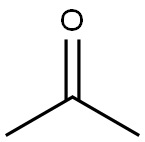
First things first, acetone is actually a polar aprotic solvent. This means it has a permanent dipole moment but doesn't donate or accept hydrogen bonds.
Now, when it comes to SN1 and SN2 reactions, polar protic solvents like water tend to stabilize carbocations, which is why they favor SN1 reactions. On the other hand, polar aprotic solvents like acetone stabilize negative charges, making them more conducive to SN2 reactions.
However, it's important to note that acetone's impact on the reaction mechanism isn't cut and dried. While it leans towards favoring SN2 reactions due to its polar aprotic nature, the specific conditions of the reaction can also play a role. For example, if the substrate is highly substituted, it might still undergo an SN1 reaction even in acetone, because the carbocation intermediate is more stable.
As for acetone's structure, its carbonyl group gives it that polar aprotic character. This makes it a good solvent for reactions involving charged species, but it doesn't stabilize carbocations as well as polar protic solvents do.
So, to sum it up, acetone is a polar aprotic solvent that tends to favor SN2 reactions over SN1 reactions. But the specific reaction conditions and the structure of the substrate can also influence which reaction mechanism prevails.
I hope that helps clear things up a bit! Let me know if you have any more questions.
First off, let's talk about the nature of acetone. Acetone is a polar aprotic solvent. It has a polar carbonyl group (C=O), which gives it some polarity, but it doesn't have an -OH or -NH group that can donate a proton, so it's aprotic.
Now, to answer your big question: Does acetone mean SN1 or SN2? Well, generally, acetone is more likely to be associated with SN2 reactions. Here's why. In SN2 reactions, the rate - determining step involves a nucleophile attacking the substrate while the leaving group departs. A polar aprotic solvent like acetone is great for SN2 reactions because it can solvate the metal cations (if there are any in the reaction mixture, like in the case of a nucleophile that comes as a salt) well. This helps to free up the nucleophile in solution. Since the nucleophile is more "free" in a polar aprotic solvent like acetone, it can attack the substrate more effectively, speeding up the SN2 reaction.
On the other hand, for SN1 reactions, the rate - determining step is the formation of a carbocation intermediate. Polar protic solvents are better for SN1 reactions because they can stabilize the carbocation through solvation. They can donate a proton and form hydrogen bonds with the leaving group as it departs. Acetone, being aprotic, can't do this as effectively as a polar protic solvent like water or ethanol. So, it doesn't really promote SN1 reactions as much.
In terms of its structure, the carbonyl group in acetone is key. The partial positive charge on the carbon of the carbonyl group can interact with the anionic part of a salt (if the nucleophile is in a salt form), separating the ions and making the nucleophile more reactive. Also, the relatively small size of the acetone molecule allows it to move around easily in the reaction mixture, which also aids in the SN2 - type interactions.
So, to sum it up, acetone doesn't "mean" SN1 or SN2 in an absolute sense, but based on its properties as a polar aprotic solvent, it has a greater impact on and is more likely to be used in SN2 reactions. I hope this gives you a better understanding of how acetone fits into the world of SN1 and SN2 reactions!
### SN1 vs. SN2: A Quick Recap
First, let’s remember the basics:
- **SN1 reactions** are two-step mechanisms where the leaving group leaves first, forming a carbocation intermediate. The nucleophile then attacks the carbocation. These reactions are favored by **polar protic solvents** (like water or alcohols) because these solvents stabilize the carbocation and the leaving group.
- **SN2 reactions** are one-step mechanisms where the nucleophile attacks the substrate at the same time the leaving group leaves. These reactions are favored by **polar aprotic solvents** (like acetone or DMSO) because these solvents don’t stabilize the nucleophile, making it more reactive.
### Where Does Acetone Fit In?
Acetone is a **polar aprotic solvent**. It has a carbonyl group (C=O), which makes it polar, but it doesn’t have any hydrogen atoms bonded to highly electronegative atoms (like O-H or N-H), so it’s not protic. This is key.
### Why Does Acetone Favor SN2 Reactions?
In SN2 reactions, the nucleophile needs to be as reactive as possible. Polar protic solvents, like water or ethanol, can hydrogen bond with the nucleophile, stabilizing it and making it less reactive. Acetone, being polar aprotic, doesn’t do this. It can solvate cations (like the leaving group) but leaves the nucleophile “naked” and ready to attack. This makes SN2 reactions faster and more favorable in acetone.
### What About SN1 Reactions?
SN1 reactions, on the other hand, rely on the stability of the carbocation intermediate. Polar protic solvents are great for this because they stabilize both the carbocation and the leaving group through hydrogen bonding and solvation. Acetone, being polar aprotic, doesn’t stabilize carbocations as effectively, so it’s not ideal for SN1 reactions.
### Acetone’s Structure and Its Role
Acetone’s structure is crucial here. The carbonyl group (C=O) is highly polar, which makes acetone a good solvent for dissolving many organic compounds. However, because it lacks O-H or N-H bonds, it can’t form hydrogen bonds with nucleophiles. This lack of hydrogen bonding is what makes it so effective for SN2 reactions—it keeps the nucleophile free and reactive.
### Practical Implications
If you’re running a reaction and want to favor SN2, acetone is a great choice. It’ll help your nucleophile stay reactive and speed up the reaction. But if you’re aiming for an SN1 reaction, you’d be better off with a polar protic solvent like ethanol or water.
### Final Thoughts
So, to answer your question: acetone favors **SN2 reactions** because it’s a polar aprotic solvent that doesn’t stabilize nucleophiles, keeping them reactive and ready to attack. It’s not ideal for SN1 reactions because it doesn’t stabilize carbocations well.
Understanding the role of solvents like acetone can really help you predict and control reaction outcomes. Keep this in mind next time you’re planning a reaction mechanism, and you’ll be golden! Hope this clears things up for you!