I'm working on a chemistry project and I'm really curious about how iron and acetone interact. I know iron is a common metal and acetone is a widely - used solvent, but I'm not sure if they actually chemically react with each other. If they do, what kind of reaction occurs? Is it a simple oxidation process, or something more complex? And does the presence of other substances, like moisture or air, affect how they interact? I'm also worried that I might misunderstand the nature of their interaction, so I’d really appreciate it if someone could shed some light on this!
How does iron interact with acetone?
Related Encyclopedia
Related Products More >
-
- 96-26-4
- CNY Request For Quotation
-
- 96-26-4
- CNY Request For Quotation
-
- CNY Request For Quotation
-
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation


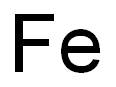

 沪ICP备2021018848号-5
沪ICP备2021018848号-5

However, if there's moisture around, that can change things. Moisture can contain dissolved oxygen, and oxygen can cause iron to oxidize. In the presence of acetone and moisture, the oxidation of iron might be a bit more complicated than just the normal rusting process. The acetone could potentially affect the rate at which the oxidation occurs, maybe by changing the surface properties of the iron or by interacting with the water molecules in some way.
If air is present along with the acetone and moisture, that also adds another factor. Air contains more oxygen, which can speed up the oxidation of iron. But it's not like there's a simple, straightforward reaction like you'd see with some other chemical combinations.
The nature of their interaction is kind of tricky. It's not a typical chemical reaction where you can easily predict the products and the process. It's more of a combination of factors, with the acetone, moisture, and air all potentially influencing how the iron behaves. So, don't worry too much if you're confused. It's a complex area, and even chemists sometimes have to do a lot of experiments to figure out exactly what's going on. Just keep in mind that while iron and acetone don't react strongly on their own, the presence of other substances like moisture and air can make the situation more interesting and complex.
That said, there are nuances. If iron has surface rust (iron oxide), acetone can help clean it by dissolving oils or contaminants clinging to the rust, but it won’t remove the rust itself. Moisture or air can indirectly play a role: if your acetone is contaminated with water (which it sometimes absorbs from the air), the water might promote iron corrosion over time, but that’s the water’s fault, not the acetone’s.
In extreme cases—like very high temperatures or with catalysts—acetone might decompose and interact with iron, but that’s not typical in labs or everyday use. For practical purposes, you can store acetone in iron containers (though stainless steel or glass is preferred to avoid any long-term moisture risks).
Bottom line? Acetone and iron ignore each other chemically. Just keep things dry to avoid side effects from water, and you’re good. No hidden reactions to worry about!
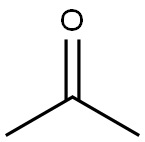
Acetone is a relatively stable organic solvent with a carbonyl group (C = O). Iron, on the other hand, is a transition metal that can participate in various redox reactions. But acetone isn't a strong enough oxidizing or reducing agent to readily react with iron in a simple, direct way at room temperature and in a dry environment.
However, if moisture is present, the situation changes. Water can react with iron to form iron(II) or iron(III) hydroxides through a corrosion process. Acetone, being a polar solvent, can dissolve some of the reaction products or act as a medium for the transport of ions. This might make the corrosion process a bit more complex. It's not a direct reaction between acetone and iron, but rather an interaction where acetone plays a secondary role in the corrosion that iron undergoes in the presence of water.
When it comes to air, oxygen from the air can also react with iron. Iron oxidizes in the presence of oxygen and moisture to form rust, which is mainly iron(III) oxide - hydroxide. Acetone might again affect this process by influencing the solubility of the reaction products or the transport of species involved in the oxidation.
It's not a simple oxidation process of acetone by iron or vice versa. Instead, it's more about how acetone, moisture, and air interact together with iron to cause potential changes. The presence of these other substances can significantly affect the nature of the interaction between iron and acetone. So, in your project, you should definitely consider the environmental conditions when studying their interaction, as they can greatly alter the outcome.