I've seen rust on old bikes, bridges, and countless metal objects, but I'm completely in the dark about what its chemical formula actually means. I know rust forms when iron reacts with oxygen, but what do the numbers and symbols in the formula tell us? Does it reveal how fast iron corrodes? Or maybe it shows the exact ratio of iron to oxygen atoms in rust? And how does understanding this formula help us prevent rust from damaging metal structures? I'm really hoping someone can break it down so I can finally grasp the significance behind rust's chemical formula.
Seeking clarity: what does rust's chemical formula mean?
Related Encyclopedia
Related Products More >
-
- CNY Request For Quotation
-
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 3564-28-1
- CNY Request For Quotation
-
- 68187-51-9
- CNY Request For Quotation


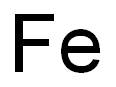

 沪ICP备2021018848号-5
沪ICP备2021018848号-5

The most common chemical formula for rust is Fe₂O₃·nH₂O. Let's break it down piece by piece. "Fe" is the symbol for iron, and you're right that rust forms when iron reacts with oxygen. The "O" stands for oxygen, and the subscript "3" means that for every two iron atoms (the "2" in Fe₂), there are three oxygen atoms. This ratio tells us how the iron and oxygen bond together to form the core part of rust, which is iron(III) oxide.
Now, that "·nH₂O" part might look a bit strange. The "n" is just a variable number, and it means that rust usually has some water molecules attached to it. That's why rust often shows up in damp environments—water helps speed up the rusting process. These water molecules can affect how rust looks and behaves. For example, they can make rust flaky and porous, which is why it tends to crumble off metal surfaces over time.
The formula doesn't directly tell us how fast iron corrodes, but it does give us clues about the end product of the corrosion process. By knowing the formula, scientists and engineers can study the structure of rust at a molecular level. They can figure out things like how the iron - oxygen bonds are arranged and how the water molecules interact with the iron(III) oxide. This knowledge helps in developing ways to prevent rust.
For instance, if we understand that water is involved in the rusting process (which the formula Fe₂O₃·nH₂O hints at), we can take steps to keep metal surfaces dry. Coating metals with paint or a protective layer of another material acts as a barrier, preventing oxygen and water from reaching the iron and thus slowing down rust formation. Also, knowing the chemical composition of rust allows us to create chemical treatments that can either dissolve existing rust or prevent new rust from forming.
So, in short, rust's chemical formula is like a code that unlocks a whole world of understanding about how iron turns into that reddish - brown substance we see on old metal. Once you get familiar with what each part of the formula means, you'll start to see how it's the key to protecting metal structures from the damaging effects of rust!
The formula Fe₂O₃ reveals two key things:
1. Atom ratios: For every 2 iron (Fe) atoms, there are 3 oxygen (O) atoms bonded together. This isn’t random—iron loses 3 electrons (becoming Fe³⁺) to oxygen, which gains them (as O²⁻), creating a stable ionic compound.
2. Structure: The atoms arrange in a repeating lattice (like a 3D grid), which explains rust’s brittleness. Those layers easily flake off, exposing fresh iron to more corrosion.
Why speed isn’t in the formula: Fe₂O₃ tells you what rust is, not how fast it forms. That depends on conditions:
• Water accelerates rust by helping electrons move (even humidity does this).
• Salt acts like a turbocharger, making rust spread faster (why ocean bridges corrode quickly).
How this helps prevent rust: Knowing rust’s formula guides protection methods:
• Barrier coatings (paint, grease): Block O₂ and H₂O from reaching iron.
• Galvanizing (zinc coatings): Zinc corrodes instead of iron (sacrificial protection).
• Alloying (stainless steel): Chromium in steel forms its own oxide layer (Cr₂O₃), which doesn’t flake off.
The formula Fe₂O₃ is like a fingerprint—it explains rust’s weakness (flakey structure) and how to outsmart it. Next time you see a rusty nail, remember: those tiny Fe₂O₃ units are why your bike frame needs that extra coat of paint!
What the formula means
Fe₂O₃: This is the core of rust—two iron (Fe) atoms bonded to three oxygen (O) atoms. It’s called iron(III) oxide because each iron atom has lost three electrons (oxidized) to form a stable compound.
·xH₂O: The “x” means rust isn’t always dry. It often traps water molecules (H₂O) in its structure, making it hydrated. The “x” varies—rust could be flaky (low x) or swollen (high x) depending on humidity.
The ratio: The formula tells us rust has 2 iron atoms for every 3 oxygen atoms. This 2:3 ratio is crucial—it’s why rust doesn’t form in a 1:1 pattern like iron oxide (FeO), a different compound.
What the formula doesn’t tell us
Corrosion speed: The formula won’t predict how fast iron rusts. That depends on environmental factors (humidity, salt, temperature). Saltwater speeds rusting because it conducts electricity, accelerating iron’s electron loss.
Structure details: Fe₂O₃·xH₂O doesn’t show rust’s crumbly, porous texture. This weakness lets oxygen and water seep in, causing more corrosion—a vicious cycle.
Why the formula matters for prevention
Understanding rust’s chemistry helps us fight it:
Block oxygen/water: Since rust needs both, coat iron with paint, oil, or a metal alloy (like stainless steel, which has chromium to form a protective oxide layer).
Cathodic protection: Use sacrificial metals (like zinc on galvanized steel). Zinc corrodes first because it’s more reactive than iron, “dying” to save the iron underneath.
Control humidity: Dehumidifiers in basements or storing metal indoors reduce the water (H₂O) in Fe₂O₃·xH₂O, slowing rust.
Add inhibitors: Chemicals like chromates or phosphates stick to iron surfaces, making it harder for oxygen to react.
Real-world example: The Golden Gate Bridge
That iconic orange isn’t just for looks—it’s a primer designed to protect the steel from rust. The bridge is also constantly repainted to seal out moisture and oxygen. Without this, the Fe₂O₃·xH₂O monster would devour it.
Final takeaway: Rust’s formula, Fe₂O₃·xH₂O, is like a recipe for corrosion—it shows the exact ratio of iron to oxygen and that water’s often involved. By tweaking the environment (blocking oxygen/water) or using protective barriers, we can starve the reaction. Next time you see rust, remember: It’s not just ugly—it’s a chemical battleground, and now you know how to win it.