I'm really scratching my head over the chemical symbol for iron. I know iron is a common element, widely used in various industries, but I have no idea why it has a specific symbol. Are chemical symbols randomly assigned, or is there a logical system behind them? Does the symbol for iron have something to do with its Latin name or its physical and chemical properties? Also, I'm curious about how this symbol is used in chemical equations and formulas. I'm hoping to get some clear and simple explanations to solve these confusions.
I'm Wondering: What Exactly Is the Chemical Symbol for the Element Iron?
Related Encyclopedia
Related Products More >
-
- 68186-88-9
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 68186-88-9
- CNY Request For Quotation
-
- 68187-51-9
- CNY Request For Quotation
-
- 68187-51-9
- CNY Request For Quotation
-
- 51274-00-1
- CNY Request For Quotation
-
- 51274-00-1
- CNY Request For Quotation


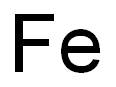

 沪ICP备2021018848号-5
沪ICP备2021018848号-5

Back in the day, when chemists started standardizing how they represented elements, they decided to use these short, simple symbols to make writing chemical formulas and equations way easier. Using the first one or two letters of the Latin name was a common way to do it. For example, sodium's symbol is Na, which comes from the Latin "natrium," and potassium is K from "kalium."
As for whether the symbol has to do with iron's properties, not directly. But once you know the symbol, it helps you understand a lot about how iron behaves in chemical reactions. In chemical equations, Fe is used just like any other element symbol. For instance, when iron reacts with oxygen to form rust (iron oxide), the equation is 4Fe + 3O₂ → 2Fe₂O₃. Here, the Fe tells you that iron atoms are involved, and the numbers in front of it and the other elements show the ratio in which they react.
These symbols are like a universal language for chemists. They make it easy to communicate complex chemical ideas. Whether you're writing a simple formula for a compound that contains iron or a really complicated equation for an industrial process that uses iron, the symbol Fe is there to represent this super important element. So, next time you see Fe in a chemistry book or lab report, you'll know it's not just some random letters - it's a nod to the element's long history and a key part of the chemical world!
Chemical symbols are usually based on the English or Latin names of the elements. For iron, the Latin name is "ferrum," and that's where the symbol Fe comes from. It's just the first two letters of the Latin word.
It's not related to iron's physical and chemical properties directly in terms of the symbol itself. But the properties of iron are important in understanding why it's used so much in different industries. Iron is strong, durable, and can conduct electricity and heat, which makes it great for things like building construction, making cars, and even in our blood in the form of hemoglobin.
In chemical equations and formulas, the symbol Fe is used to represent iron. For example, if you have a compound like iron oxide, which is a common form of rust, its chemical formula is Fe₂O₃. This tells you that there are two iron atoms (Fe) and three oxygen atoms (O) in each molecule of iron oxide.
Chemical symbols like Fe are really useful because they allow scientists to write out chemical reactions and formulas in a short and easy - to - understand way. Instead of writing out the full name of the element every time, they can just use the symbol.
So, in summary, the chemical symbol for iron (Fe) comes from its Latin name "ferrum." It's used in chemical equations and formulas to represent iron atoms, and it's part of a system that makes it easy for scientists to communicate about chemical reactions.
This system of using Latin - based symbols helps to standardize the naming of elements across different languages. It doesn't have a direct relation to iron's physical and chemical properties, but it provides a consistent way to represent the element in scientific communication.
In chemical equations and formulas, the symbol "Fe" is used to represent iron atoms or ions. For example, in the compound iron oxide (rust), the formula is Fe₂O₃. Here, the "Fe" indicates that iron is a part of the compound, and the subscript "2" shows that there are two iron atoms for every three oxygen atoms. In a chemical equation like the reaction between iron and sulfuric acid: Fe + H₂SO₄ → FeSO₄ + H₂↑, the "Fe" represents the iron metal that reacts with sulfuric acid to form iron sulfate and hydrogen gas.
The use of symbols like "Fe" makes it much easier to write and understand chemical equations and formulas. Instead of writing out the full name of the element every time, chemists can use these concise symbols. It also allows for a more universal understanding of chemical reactions and compounds, regardless of the language spoken by the chemist. So, the symbol "Fe" is an important part of the language of chemistry, with its roots in the Latin name of the element and a crucial role in representing iron in chemical equations and formulas.